Matter can exist in three states: solid, liquid, and gas, depending on temperature and pressure. This transition between states is known as the “three states of matter” and is fundamental in both physics and chemistry. In this article, we will explain the three states of matter, the movement of molecules, everyday examples, and useful points for preparing for the hazardous materials handler exam.
- Basic Concept of the Three States of Matter
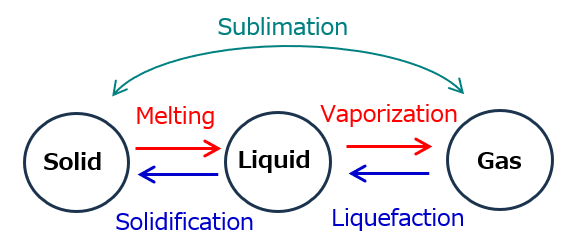
- Specific Changes in the Three States of MatterState changes occur due to energy transfer. Changes in temperature and pressure cause matter to transition between solid, liquid, and gas. Below are the main processes of state changes:
- Everyday Examples of the Three States of Matter
- The Role of the Three States of Matter in the Hazardous Materials Exam
- Example Exam Question 1
- Example Exam Question 2
- Conclusion
Basic Concept of the Three States of Matter
The three states of matter refer to solid, liquid, and gas. Matter changes between these states depending on temperature and pressure. For example, ice (solid water) melts into water (liquid), and further heating causes it to evaporate into water vapor (gas). These state changes are significantly influenced by the structure of matter and the movement of molecules.
Molecular Movement in Solids, Liquids, and Gases
In solids, molecules are tightly bound and arranged in a regular pattern. As temperature increases, these molecules start vibrating more vigorously. When heated further, the molecules gain enough energy to break free from their fixed positions, turning into a liquid. In a liquid, molecules move more freely but are still somewhat bound by intermolecular forces. As the temperature continues to rise, the molecules gain enough energy to completely overcome these forces, transitioning into a gas where they move freely through space.
Specific Changes in the Three States of MatterState changes occur due to energy transfer. Changes in temperature and pressure cause matter to transition between solid, liquid, and gas. Below are the main processes of state changes:
- Melting (solid → liquid): For example, ice melts into water when it absorbs heat.
- Freezing (liquid → solid): Water freezes into ice as it releases heat.
- Evaporation (liquid → gas): Water turns into vapor when it absorbs heat.
- Condensation (gas → liquid): Water vapor condenses into liquid when heat is released.
- Sublimation (solid → gas, gas → solid): Dry ice sublimates directly from solid to gas, while frost forms when water vapor sublimates directly into solid ice.
Everyday Examples of the Three States of Matter
The three states of matter can easily be observed in everyday life. Here are a few common examples:
- Ice, Water, and Water Vapor: Water can exist as solid ice, liquid water, and gas (steam) depending on the temperature.
- Dry Ice: Dry ice (solid carbon dioxide) sublimates directly into gas without becoming liquid.
- Steam and Condensation: Water vapor in the air condenses into liquid droplets when cooled, a process known as condensation.
The Role of the Three States of Matter in the Hazardous Materials Exam
The hazardous materials handler exam frequently includes questions about the three states of matter. Understanding how matter behaves in different states and which states are more dangerous when handling hazardous materials is critical. Here are some important points for exam preparation:
Key Points for Exam Preparation
- Energy Transfer: Understand the energy absorption and release involved in state changes such as melting and evaporation.
- Properties of Hazardous Materials: Recognize how the state of a hazardous material (solid, liquid, or gas) affects its risk and management.
- Accurate Use of Terms: Master the correct usage of terms like “melting,” “evaporation,” and “condensation” for exam success.
Example Exam Question 1
次のうち、物質が固体から直接気体に変わる現象を何というか。
(1) 蒸発
(2) 凝固
(3) 融解
(4) 昇華
(5) 液化
Which of the following describes a process where a substance changes directly from a solid to a gas?
(1) Evaporation
(2) Freezing
(3) Melting
(4) Sublimation
(5) Liquefaction
Explanation:
The process where a substance changes directly from a solid to a gas is called “sublimation.” A typical example is dry ice, which sublimates directly from solid to gas.
Answer: (4)
Example Exam Question 2
物質の三態についての記述で、次のうち誤っているのはどれか。
(1) 固体と液体と気体の3つの状態を、物質の三態という。
(2) 固体が液体に代わる変化を融解という。
(3) 凝縮または液化とは、気体が液体に変化することでドライアイスが溶けるのがその例である。
(4) 昇華とは、ナフタリンが蒸気になるように、固体から気体に直接変化することや、その逆に気体から固体に直接変化することをいう。
(5) 液体が固体に代わることを凝固という。
Which of the following statements about the three states of matter is incorrect?
(1) Solid, liquid, and gas are known as the three states of matter.
(2) The change from solid to liquid is called melting.
(3) Condensation or liquefaction refers to the change from gas to liquid, with dry ice melting as an example.
(4) Sublimation refers to the direct change from solid to gas, like naphthalene evaporating, or the reverse process of gas changing directly to solid.
(5) The process of liquid turning into solid is called freezing.
Explanation:
The process described in option (3) is incorrect. Dry ice does not melt; it sublimates, meaning it transitions directly from a solid to a gas.
Answer: (3)
Conclusion
The three states of matter are fundamental knowledge for the hazardous materials handler exam. Understanding state changes, molecular movement, and energy transfer will help you prepare thoroughly for the exam.


コメント